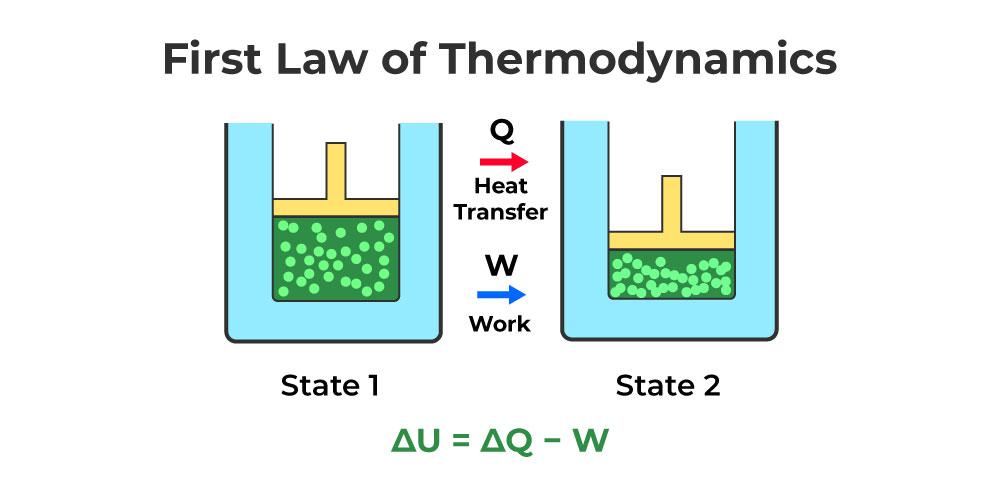
The First Law of Thermodynamics, also known as the Law of Energy Conservation, is a fundamental principle in physics and engineering that governs the behavior of energy in thermodynamic systems. This law is central to all branches of science and engineering, influencing our understanding of everything from internal combustion engines to biological systems.
Definition of the First Law of Thermodynamics
The First Law of Thermodynamics states that:
Energy can neither be created nor destroyed; it can only be transformed from one form to another.
In mathematical terms, the law is expressed as:
ΔU = Q – W
Where:
- ΔU = Change in internal energy of the system
- Q = Heat added to the system
- W = Work done by the system
This equation highlights that the internal energy of a closed system changes due to heat transfer and work interactions.
Concept of Internal Energy
Internal energy (U) is the total energy contained within a system due to the kinetic and potential energies of its molecules. It includes:
- Translational energy
- Rotational energy
- Vibrational energy
- Intermolecular forces
When heat is added or work is performed on the system, the internal energy increases. Conversely, when the system performs work or loses heat, its internal energy decreases.
Forms of Energy Transfer
The First Law of Thermodynamics concerns two primary modes of energy transfer:
1. Heat (Q)
Heat is energy transferred due to temperature differences between systems. It always flows from a hotter object to a cooler one, and it is measured in joules (J) or calories (cal).
- Positive Q means heat is added to the system.
- Negative Q means heat is removed from the system.
2. Work (W)
Work is energy transferred when an object is moved by a force. In thermodynamics, work is typically done by the system on its surroundings, such as gas expansion in a piston.
- Positive W indicates the system does work on the surroundings.
- Negative W indicates work is done on the system.
Application to Closed Systems
In a closed system, mass remains constant, but energy may be exchanged with the surroundings via heat and work.
Example:
Consider a gas in a piston-cylinder assembly. When heat is added, the gas expands, doing work on the piston. The internal energy changes according to the equation:
ΔU = Q – W
If no heat is added (Q = 0), then any work done by the system must result in a decrease in internal energy.
Application to Cyclic Processes
In a thermodynamic cycle, the system returns to its original state, so the change in internal energy is zero:
ΔU = 0 → Q = W
This means that in a cyclic process, the net heat added equals the net work done by the system over the entire cycle.
This principle is essential in heat engines, such as steam turbines or internal combustion engines.
Real-World Examples of the First Law of Thermodynamics
1. Internal Combustion Engine
In car engines:
- Fuel combustion adds heat (Q) to the system.
- The expanding gases do work (W) on the piston.
- The internal energy changes as heat and work are exchanged, following ΔU = Q – W.
2. Refrigerators
Refrigerators operate by removing heat from the inside and expelling it to the surroundings:
- Work is done by the compressor.
- Heat is removed from the interior (negative Q).
- The law ensures that the energy input equals the heat removed plus the work done.
3. Steam Turbines
In a power plant:
- Steam generated in boilers adds heat to the system.
- The steam does work by rotating turbines.
- The efficiency is determined by how well heat is converted into work, governed by the First Law.
First Law vs. Other Laws of Thermodynamics
While the First Law of Thermodynamics addresses energy conservation, it does not account for the directionality or quality of energy. For that, we turn to:
- The Second Law, which introduces entropy and irreversibility.
- The Third Law, which defines behavior at absolute zero.
- The Zeroth Law, which defines thermal equilibrium.
Together, these laws form the foundation of classical thermodynamics.
Significance in Engineering and Science
The First Law is a cornerstone principle in fields such as:
- Mechanical Engineering: Used in heat engine design, HVAC systems, and turbines.
- Chemical Engineering: Applies to energy balances in chemical reactors.
- Physics: Underlies concepts of conservation in closed systems.
- Biology: Helps explain metabolic energy conversion in living organisms.
- Astronomy: Applied in modeling the thermodynamic behavior of stars.
Limitations and Misconceptions
1. Cannot Predict Energy Quality
The First Law tells us energy is conserved, but not whether it is useful. For example:
- Heat energy lost in friction is conserved but not recoverable for work.
2. Direction of Processes
The law does not indicate which direction a process will occur (e.g., heat flows from hot to cold), which is addressed by the Second Law.
3. Perpetual Motion Machines
Some erroneously believe it’s possible to design a perpetual motion machine of the first kind, which violates the First Law by generating energy without input. This is scientifically impossible.
Mathematical Derivations and Practical Use
For ideal gases:
dU = nC_v dT
Where:
- n = number of moles
- C_v = molar heat capacity at constant volume
- dT = temperature change
This relation is used in calculating the internal energy change during thermodynamic processes such as:
- Isothermal process: ΔU = 0
- Adiabatic process: Q = 0, so ΔU = –W
- Isochoric process: W = 0, so ΔU = Q
- Isobaric process: ΔU = Q – PΔV
Engineers use these equations to design more efficient systems.
Conclusion
The First Law of Thermodynamics is one of the most essential and universal principles in all of science. It explains how energy flows, transforms, and remains conserved in closed systems, regardless of the complexity or scale. Whether designing power plants, analyzing engines, or understanding biological metabolism, this law ensures that energy is always accounted for.
Its significance spans every scientific discipline and serves as the foundation for modern engineering, environmental science, and thermodynamic modeling.